- Model NO.: BDH05
- Material: Steel
- Install: Simple
- Trademark: JNK
- Specification: Q235
- Surface Treatment: Zinc Plated
- How to Used: Barn Door for Outside and Interier
- Delivery Date: 8-20 Days
- Transport Package: Polybag First, Then Carton and Pallet
- Origin: China
1.Steel Flat Track (2M)-
2.Hangers roller  ;Steel bodied hangers with solid nylon wheels
3.Track BracketsÂ
4.Installation Hardware
5.Door Bottom Guide
6.Track Stops Mounting
7.Anti-rise Discs
Features and funtion
1. Surface: Black powder coated for corrosion protection
2. Main Material: High quality Q235 steel+POM materials
3. Passed the test of opening - closing: 120000 times
4. Â Used For:barn door,Kitchen, dinning room, bedroom, coffee bar, farm, Â window etc.
| Door panel thickness | 35/40mm |
| Door Max Weight:Â | Â 150lb - 200lb |
| 6FT-16FT Track fit for door opening | Â 36inch - 96inch |
Design Reference

Â
Package and shipping :


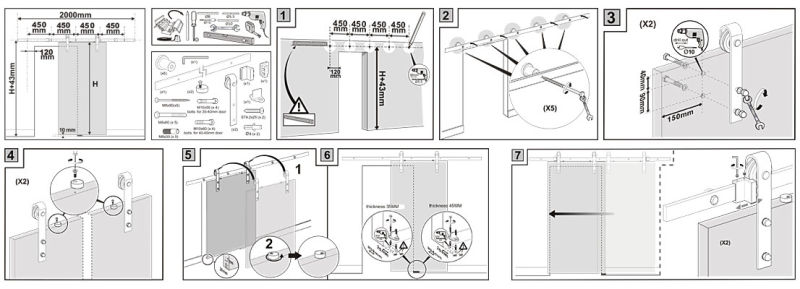
Installation step for Sliding flat track roller usd in Interier doors
Jiaxing Jinnaike Hardware Products Co. Ltd., a leading worldwide manufacturer in China since originally1995, specializes in manufacturing gate, door, windows and fence's accessories, sliding gate pulley , hardware which is used for , swing gate, sliding cantilever gate, sliding door, folding door and electric retractable door, fence, farm etc.

   Jiaxing Jinnaike Hardware Products Co. Ltd., has been certified to comply with ISO9001:2008 Quality Management System, BV(Bureau Veritas) and SGS.
If any interests, please give me a message for more details, thank you!
Dioxaborolane Series is widely used in Suzuki reaction
The Suzuki reaction is an organic reaction, classified as a coupling reaction, where the coupling partners are a boronic acid and an organohalide catalyzed by a palladium(0) complex. It was first published in 1979 by Akira Suzuki and he shared the 2010 Nobel Prize in Chemistry with Richard F. Heck and Ei-ichi Negishi for their effort for discovery and development of palladium-catalyzed cross couplings in organic synthesis. In many publications this reaction also goes by the name Suzuki–Miyaura reaction and is also referred to as the Suzuki coupling. It is widely used to synthesize poly-olefins, styrenes, and substituted biphenyls. Several reviews have been published describing advancements and the development of the Suzuki Reaction.[5][6][7] The general scheme for the Suzuki reaction is shown below where a carbon-carbon single bond is formed by coupling an organoboron species (R1-BY2) with a halide (R2-X) using a palladium catalyst and a base.
Dioxaborolane Series
Dioxaborolane Series,214360-69-7 Large Scale,214360-69-7 In Stock,1356963-11-5 Large Scale
Jinan Meigao Biopharmaceutical Technology Co., Ltd. , http://www.meigaobiopharma.com